Elyse Betters Picaro/ZDNET
Follow ZDNET: Add us as a preferred source on Google.
ZDNET’s key takeaways
- Withings’ BeamO smart thermometer received FDA clearance Thursday.
- It can take your body temperature and data on heart and lung health.
- It’s available on Withings’ website and coming to other retailers next year.
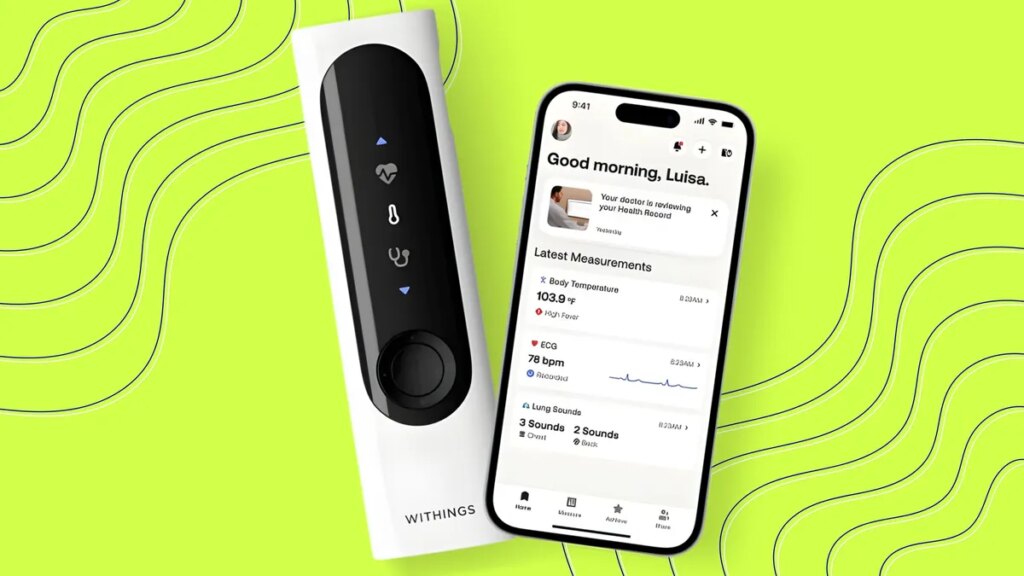
The latest thermometers are ditching mercury and replacing it with high-tech sensors instead. One smart thermometer, developed by French health technology company Withings, received FDA clearance on Thursday.
The Withings BeamO “thermometer of the future” not only takes a person’s temperature. It can also perform a medically certified electrocardiogram and an auscultation for monitoring heart and lung health in less than a minute.
Also: The next frontier of health tracking is happening in your toilet
“BeamO brings access to key vital signs, typically measured during medical consultations, into everyday life. All this new data on the heart, lungs, and temperature provides an overview of the state of each user’s health,” said Eric Carreel, Withings founder and president, in a press release.
When a person takes their temperature with BeamO, the sensors within the device record their heart’s electrical activity, measure infrared light for body temperature, and capture acoustic waves to interpret heart and lung activity. This data is translated to the app and interpreted by AI to detect variations from a person’s baseline.
Also: This new Withings smartwatch can tell you when you’re getting sick
The thermometer is one of the many devices in Withings’ ecosystem that connects to Withings’ HealthLink. This integrated feature in the Withings app creates links and reports of health data that people can send to a clinician for review.
Earlier this year, Withings unveiled Cardio Check-Up, a feature available through the paid Withings+ membership tier that provides access to a board-certified cardiologist for review of cardiac health assessments in less than 24 hours.
“While BeamO doesn’t diagnose, recording lung sounds and temperature over time, especially during the winter season when viral infections are more prevalent, can help doctors assess respiratory conditions more effectively,” Withings said in the press release.
Where to get BeamO
BeamO is now available to purchase directly from Withings and will be available on other retailers, including Amazon, in 2026. Upon purchase of BeamO, customers also receive a complimentary one-month subscription to Withings+ and one free Cardio Check-Up appointment.
When a device receives FDA clearance, this means that the new medical device has proven to be “substantially equivalent” to existing devices marketed for the same use, the FDA explains. Companies seeking FDA clearance are required to submit information proving that their device is on par with existing devices of the same standard.